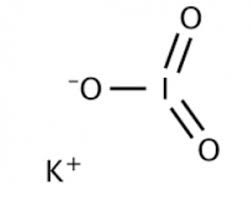
Potassium Iodate

Product Description
Potassium iodate, a chemical compound consisting of potassium, iodine, and oxygen atoms, has several industrial applications.
Product:
Potassium Iodate
CAS:
7758-05-6
Synonym:
Potassium triodate; Iodic acid, potassium salt
Structure:
Typical Characteristics
Appearance
White crystalline powder
Density
3.93 g/cm3
Melting point
560 °C
Molecular Weight
214.00
Odor
Odorless
Purity
99.5%
Uses, Applications & Markets
Key applications
Markets
get a quote
We Offer Potassium Iodate
in various grades
A few of the grades available are listed below:


Potassium Iodate used in many
industry applications
Potassium iodate, a chemical compound consisting of potassium, iodine, and oxygen atoms, has several industrial applications. Here are some of its uses:
- Food Additive: Potassium iodate is utilized as a food additive, primarily in salt for iodization. It helps prevent iodine deficiency disorders (IDDs) by ensuring an adequate intake of iodine, which is essential for thyroid hormone synthesis and overall health.
- Water Treatment: In water purification processes, potassium iodate can be used to disinfect and sanitize drinking water. It helps eliminate harmful microorganisms, such as bacteria, viruses, and parasites, making water safe for consumption.
- Laboratory Reagent: Potassium iodate serves as a laboratory reagent in chemical analysis, titration, and iodometric methods. It is used to determine the concentration of reducing agents, such as sulfites and thiosulfates, in various samples.
- Pharmaceuticals: Potassium iodate may be employed in pharmaceutical formulations for its antiseptic and germicidal properties. It can be found in topical antiseptic solutions, wound cleansers, and oral hygiene products to prevent infections.
- Chemical Synthesis: Potassium iodate is used as a source of iodine in organic synthesis reactions, particularly in the production of pharmaceuticals, dyes, and fine chemicals. It serves as a precursor for the preparation of various iodinated compounds.
- Photography: In the past, potassium iodate was used in certain photographic processes as a component of developer solutions. However, its use in photography has declined with the advent of digital photography and alternative chemicals.