HI! I’M ELEMENT AI.
Tris-HCl

Product Description
Tris-HCl is a commonly used buffer in biochemistry and molecular biology.
Product:
Tris-HCl
CAS:
1185-53-1
Synonym:
TRIS hydrochloride; Tris-(Hydroxymethyl)-aminomethane hydrochloride; Trizma® hydrochloride
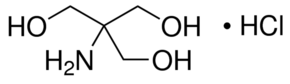
Structure:
Typical Characteristics
Appearance
White Powder
Density
1.05 g/cm3
Melting point
150 - 152 °C
Molecular Weight
157.60
Purity
≧ 99.0%
Uses, Applications & Markets
Key applications
Markets
get a quote


Tris-HCl used in many
industry applications
Tris-HCl, also known as tris(hydroxymethyl)aminomethane hydrochloride, is a commonly used buffer in biochemistry and molecular biology. Here's a list of some of its uses:
- Buffer Solution: Tris-HCl is widely used as a buffering agent in various biological and biochemical applications, such as protein and nucleic acid research. It helps maintain a stable pH environment, typically around pH 7.0 to 9.0, which is suitable for many enzymatic reactions and cell culture studies.
- Protein Purification: It is employed in protein purification procedures, such as chromatography and gel electrophoresis, to stabilize protein samples and maintain optimal pH conditions for protein stability and activity.
- Cell Culture: Tris-HCl buffer is used in cell culture media to maintain the pH of the growth environment and provide a stable osmotic environment for cell growth and proliferation.
- Molecular Biology: It is utilized in molecular biology techniques, including DNA and RNA isolation, amplification (PCR), and sequencing, where maintaining a precise pH is crucial for enzymatic reactions and nucleic acid stability.
- Enzyme Kinetics: Tris-HCl buffer is used in enzyme kinetics studies to control the pH of the reaction mixture and maintain enzyme activity over a range of pH values.
- Electrophoresis: It is used in gel electrophoresis techniques, such as SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), for separating proteins and nucleic acids based on size and charge.
- Immunohistochemistry: Tris-HCl buffer is employed in immunohistochemical staining procedures to provide a stable pH environment for antigen-antibody interactions and enzyme-substrate reactions.
- Diagnostic Assays: Tris-HCl buffer is used in diagnostic assays, such as enzyme-linked immunosorbent assays (ELISA) and Western blotting, to maintain optimal pH conditions for antigen-antibody binding and detection.
- Drug Formulation: It is used in pharmaceutical formulations to adjust the pH of drugs and drug delivery systems, ensuring stability and compatibility with physiological conditions.
- Microbiology: Tris-HCl buffer is utilized in microbiology techniques for bacterial and fungal culture media preparation, where it helps maintain the pH of the growth medium for optimal microbial growth and isolation.