HI! I’M ELEMENT AI.
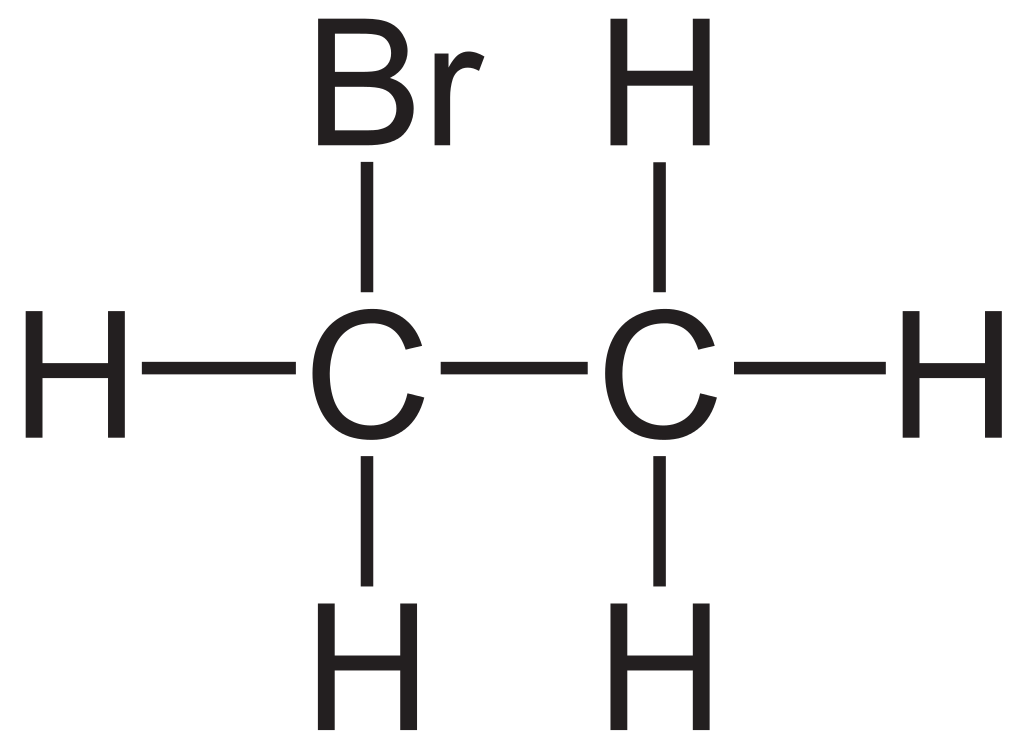
Ethyl Bromide

Product Description
Ethyl Bromide, also known as bromoethane, is a chemical compound with various industrial applications.
Product:
Ethyl Bromide
CAS:
74-96-4
Synonym:
Bromoethane; Monobromoethane; 1-Bromoethane
Structure:
Typical Characteristics
Appearance
Colorless liquid
Boiling point
38 °C
Density
1.46 g/cm3
Flash Point
-20 °C
Melting point
-119 °C
Molecular Weight
108.97
Odor
Ether-like
Purity
99%
Refractive index
1.4225
Uses, Applications & Markets
Key applications
get a quote
We Offer Ethyl Bromide
in various grades
A few of the grades available are listed below:


Ethyl Bromide used in many
industry applications
Ethyl Bromide, also known as bromoethane, is a chemical compound with various industrial applications. Here are some of its uses:
- Organic Synthesis: Ethyl Bromide is used as a reagent in organic synthesis reactions to introduce ethyl groups into organic molecules. It serves as an alkylating agent in the production of pharmaceuticals, agrochemicals, and fine chemicals.
- Solvent: It can act as a solvent for various organic compounds, particularly in specialized chemical processes and reactions. Ethyl Bromide is also used as a solvent for cleaning and degreasing metal surfaces.
- Fumigant: Ethyl Bromide has been historically used as a fumigant for pest control in agriculture, storage facilities, and quarantine treatments. However, its use as a fumigant has declined due to environmental and health concerns.
- Intermediate: It serves as an intermediate in the production of other organic compounds, such as ethylamines, ethyl esters, and ethyl ethers. These compounds find applications in various industries, including pharmaceuticals, polymers, and flavors.
- Pharmaceuticals: Ethyl Bromide has been used in the past as an ingredient in pharmaceutical formulations, particularly as a sedative and analgesic. However, its medical use has largely been discontinued due to safety issues and the availability of safer alternatives.
- Lab Reagent: In laboratory settings, Ethyl Bromide may be used as a reagent for specific chemical reactions or as a precursor for the synthesis of other compounds. However, its use in laboratories is limited due to its toxic and hazardous nature.
- Fire Extinguishing Agent: Ethyl Bromide was once considered as a fire extinguishing agent, particularly for extinguishing electrical fires. However, its use for this purpose has been largely discontinued due to safety concerns and the availability of more effective alternatives.