Strontium Chloride

Product Description
Strontium chloride, a salt of strontium and chloride ions, possesses several industrial applications due to its distinctive properties.
Product:
Strontium Chloride
CAS:
10025-70-4
Synonym:
Strontium dichloride hexahydrate
Structure:
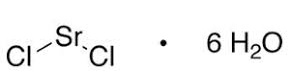
Typical Characteristics
Appearance
White crystalline powder
Density
1.930 g/cm3
Melting point
115 °C
Molecular Weight
266.62
Odor
Odorless
Purity
99%
Refractive index
1.536
Uses, Applications & Markets
Key applications
get a quote
We Offer Strontium Chloride
in various grades
A few of the grades available are listed below:


Strontium Chloride used in many
industry applications
Strontium chloride, a salt of strontium and chloride ions, possesses several industrial applications due to its distinctive properties. Here are some of its key industrial uses:
- Pyrotechnics: Strontium chloride is commonly employed as a pyrotechnic colorant to produce red flames in fireworks and flares. When burned, strontium ions emit a characteristic red-orange light, making it a popular choice for creating vibrant red hues in pyrotechnic displays.
- Manufacturing: It serves as a precursor in the manufacturing of various strontium compounds, including strontium carbonate and strontium hydroxide, which are utilized in the production of ceramic materials, glass, ferrite magnets, and fluorescent lights. Strontium chloride is often used as a starting material for synthesizing these compounds.
- Chemical Synthesis: Strontium chloride finds application as a catalyst or reagent in organic synthesis reactions, particularly in the production of pharmaceuticals, agrochemicals, and specialty chemicals. It may be employed in various transformations, such as Friedel-Crafts acylation, halogenation, and Grignard reactions.
- Dental Applications: In dentistry, strontium chloride is utilized in toothpaste formulations and mouthwash solutions for its desensitizing properties. It helps alleviate tooth sensitivity by blocking nerve signals in the teeth, providing relief from discomfort caused by hot or cold stimuli.
- Medical Imaging: Strontium chloride has been investigated for potential applications in medical imaging, particularly in positron emission tomography (PET) imaging. Radioactive isotopes of strontium, such as strontium-82, can be used as tracers for studying bone metabolism and diagnosing bone-related diseases.
- Heat Treatment: In metallurgy, strontium chloride is sometimes employed as an additive in heat treatment processes for ferrous and non-ferrous metals. It can modify the microstructure of metal alloys, improve mechanical properties, and enhance surface hardness and wear resistance through grain refinement.
- Biomedical Research: Strontium chloride is utilized in biomedical research studies to investigate its effects on bone health and bone tissue engineering. It has shown potential for promoting bone formation and bone regeneration, making it a subject of interest in the development of orthopedic implants and biomaterials.
- Water Treatment: Strontium chloride may be used in water treatment applications for its ability to remove sulfate ions and alkalinity from water sources. It can help reduce scaling and fouling in industrial processes and water distribution systems, improving water quality and system efficiency.