HI! I’M ELEMENT AI.
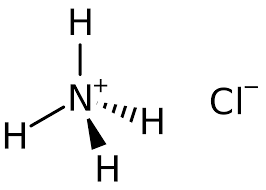
Ammonium Chloride

Product Description
Ammonium Chloride, also known as sal ammoniac, is an inorganic compound with various industrial applications.
Product:
Ammonium Chloride
CAS:
12125-02-9
Synonym:
Sal ammoniac
Structure:
Typical Characteristics
Appearance
White crystalline solid
Boiling point
520 ºC
Density
1.53 g/cm3
Melting point
340 °C
Molecular Weight
53.49
Odor
Odorless
Purity
≥99.5%
Refractive index
1.642
Uses, Applications & Markets
Key applications
get a quote
We Offer Ammonium Chloride
in various grades
A few of the grades available are listed below:


Ammonium Chloride used in many
industry applications
Ammonium chloride is a chemical compound with various industrial applications. Here's a list of some of its uses:
- Fertilizers: Ammonium chloride is commonly used as a nitrogen fertilizer in agriculture. It provides a readily available source of nitrogen for plants, promoting healthy growth and increasing crop yields. It is particularly useful for crops that require nitrogen in the early stages of growth.
- Pharmaceuticals: It is used in the pharmaceutical industry as an expectorant and cough suppressant in cough syrups and cold medications. Ammonium chloride helps to thin and loosen mucus in the respiratory tract, making it easier to cough up and expel.
- Electroplating: Ammonium chloride is used in electroplating baths as an electrolyte and flux. It helps to provide a conductive solution for the deposition of metal coatings on substrates, such as steel, copper, and aluminum. It also helps to remove oxides and contaminants from the metal surface, ensuring a clean and uniform coating.
- Leather Tanning: It is used in the leather industry as a tanning agent and pH regulator in the tanning process. Ammonium chloride helps to remove hair and other unwanted materials from animal hides, as well as to stabilize the pH of the tanning solution, ensuring proper penetration of tanning agents.
- Textile Printing: Ammonium chloride is used in textile printing as a mordant and fixing agent for dyes. It helps to improve the adhesion and colorfastness of dyes to the fabric, ensuring vibrant and long-lasting prints. It is particularly useful for printing on cotton and other cellulosic fibers.
- Batteries: It is used in dry cell batteries as an electrolyte and depolarizer. Ammonium chloride helps to conduct electricity between the battery electrodes and prevent polarization, ensuring efficient and reliable battery performance. It is commonly used in zinc-carbon batteries and manganese dioxide batteries.
- Fire Extinguishers: Ammonium chloride is used in some dry powder fire extinguishers as a fire suppressant. When heated, it decomposes to release ammonia and hydrogen chloride gases, which react with the flames to suppress combustion. It is effective for extinguishing fires involving wood, paper, textiles, and flammable liquids.
- Adhesives: It is used in the production of adhesives and glues as a resin modifier and curing agent. Ammonium chloride helps to improve the adhesion, viscosity, and curing rate of adhesive formulations, making them suitable for bonding a wide range of substrates, including wood, metal, and plastics.
- Galvanizing: Ammonium chloride is used in the galvanizing industry as a flux for cleaning and preparing metal surfaces prior to galvanization. It helps to remove oxides, grease, and other contaminants from the metal surface, ensuring proper adhesion and coverage of the zinc coating.
- Laboratory Reagent: Ammonium chloride is used as a laboratory reagent and analytical standard in chemical analysis and research. It is used in various analytical techniques, such as titrations, chromatography, and spectrophotometry, for the quantitative determination of cations and anions in samples.