HI! I’M ELEMENT AI.
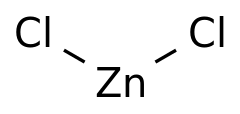
Zinc Chloride

Product Description
Zinc chloride, with the chemical formula ZnCl2, finds various industrial applications owing to its unique properties.
Product:
Zinc Chloride
CAS:
7646-85-7
Synonym:
Dichlorozinc; Zinc dichloride
Structure:
Typical Characteristics
Appearance
White crystalline powder
Boiling point
732 °C
Density
2.907 g/cm3
Melting point
290 °C
Molecular Weight
136.30
Odor
Odorless
Purity
≥99%
Refractive index
1.681
Uses, Applications & Markets
Key applications
get a quote
We Offer Zinc Chloride
in various grades
A few of the grades available are listed below:


Zinc Chloride used in many
industry applications
Zinc chloride, with the chemical formula ZnCl2, finds various industrial applications owing to its unique properties. Here's a list of some of its industrial uses:
- Galvanizing: Zinc chloride is used as an activator in the galvanizing process, where it helps prepare metal surfaces for the deposition of zinc coatings. It removes oxide layers and contaminants from steel surfaces, promoting adhesion and ensuring uniform coverage of the zinc layer. Galvanized steel is widely used in construction, automotive, and industrial applications for corrosion protection.
- Fluxes: Zinc chloride is employed as a flux in soldering, brazing, and welding applications. It removes metal oxides and surface impurities, facilitating the wetting and bonding of metals during joining processes. Zinc chloride fluxes are used in electronics assembly, plumbing, metal fabrication, and jewelry making to improve solder flow and joint strength.
- Wood Preservation: Zinc chloride is used as a wood preservative to protect timber against fungal decay and insect damage. It is applied to pressure-treated lumber or impregnated into wood products to extend their service life in outdoor environments. Zinc chloride penetrates deep into the wood fibers, providing long-lasting protection against rot, mold, and termite infestation.
- Deodorants: Zinc chloride is utilized in antiperspirant and deodorant formulations to control perspiration and odor. It acts as an astringent, temporarily reducing the size of sweat glands and decreasing sweat production. Zinc chloride-based antiperspirants are applied topically to the skin, providing long-lasting protection against underarm wetness and body odor.
- Chemical Synthesis: Zinc chloride serves as a catalyst or reagent in various chemical synthesis reactions. It is used in organic synthesis for Friedel-Crafts acylation, Beckmann rearrangement, and other transformations. Zinc chloride can facilitate the formation of carbon-carbon and carbon-heteroatom bonds in the production of pharmaceuticals, agrochemicals, and specialty chemicals.
- Electroplating: Zinc chloride is employed in electroplating baths as a source of zinc ions for depositing metallic zinc coatings on metal substrates. Electroplated zinc coatings provide corrosion protection and decorative finishes for steel, iron, and other metals. Zinc chloride-based plating solutions are used in automotive parts, hardware, fasteners, and architectural components.
- Textile Processing: Zinc chloride is used in textile processing as a mordant or dye fixative to improve the colorfastness of dyed fabrics. It helps bind textile dyes to fibers and enhances their resistance to fading, washing, and light exposure. Zinc chloride mordants are applied in natural dyeing processes to achieve vibrant and long-lasting coloration of textiles.
- Battery Electrolytes: Zinc chloride solutions are employed as electrolytes in certain types of batteries, such as zinc-carbon and zinc-chloride batteries. They facilitate the flow of ions between the electrodes during battery discharge and recharge cycles. Zinc chloride-based electrolytes provide good conductivity and chemical stability, contributing to the performance and efficiency of the batteries.
- Oil and Gas Industry: Zinc chloride is used in the oil and gas industry as a completion fluid or workover fluid in drilling operations. It helps control formation pressures, prevent wellbore collapse, and remove drilling debris from boreholes. Zinc chloride brines are also utilized in hydraulic fracturing ("fracking") to improve fluid viscosity and proppant transport in shale formations.
- Adhesives: Zinc chloride is employed as a curing agent or activator in certain types of adhesives and sealants. It promotes cross-linking reactions between polymer chains, leading to the formation of strong and durable bonds. Zinc chloride-based adhesives are used in automotive assembly, construction, electronics, and aerospace applications for bonding metals, plastics, and composites.