Lithium Battery & Electrolyte Chemicals
HI! I’M ELEMENT AI.
Nickel Sulfate

Product Description
Nickel sulfate is a chemical compound with various industrial, commercial, and research applications.
Product:
Nickel Sulfate
CAS:
10101-97-0
Synonym:
Nickel(II) sulfate; Nickel sulfate hexahydrate
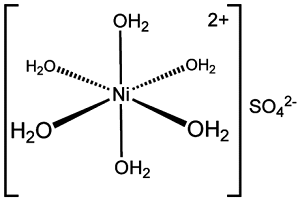
Structure:
Typical Characteristics
Appearance
Blue to emerald-green crystals
Boiling point
100 °C (decomposes)
Density
2.07 g/cm3
Melting point
53 °C
Molecular Weight
262.85
Odor
Odorless
Purity
≥98%
Refractive index
1.511
Uses, Applications & Markets
Key applications
Markets
get a quote
We Offer Nickel Sulfate
in various grades
A few of the grades available are listed below:


Nickel Sulfate used in many
industry applications
Nickel sulfate is a chemical compound with various industrial, commercial, and research applications. Here are some of its uses:
- Electroplating: Nickel sulfate is primarily used in the electroplating industry for the deposition of nickel metal onto surfaces. It serves as an essential electrolyte in nickel plating baths, where it provides nickel ions for the electrodeposition process. Nickel-plated coatings offer corrosion resistance, wear resistance, and decorative finishes on metal substrates.
- Catalysis: Nickel sulfate is used as a catalyst in various chemical reactions, particularly in organic synthesis and hydrogenation reactions. It may be employed in the production of pharmaceuticals, fine chemicals, and specialty compounds where nickel complexes act as catalysts to facilitate desired transformations.
- Battery Manufacturing: Nickel sulfate is used in the production of rechargeable nickel-cadmium (Ni-Cd) batteries and nickel-metal hydride (NiMH) batteries. It serves as a key component in the positive electrode (cathode) of these batteries, where it helps store and release electrical energy during charge and discharge cycles.
- Electronics Industry: Nickel sulfate is used in the electronics industry for various applications such as electroforming, printed circuit board (PCB) manufacturing, and semiconductor fabrication. It is used to deposit thin layers of nickel onto substrates for electrical contacts, solder masks, and surface finishes in electronic devices.
- Surface Treatment: Nickel sulfate is used in surface treatment processes such as metal finishing, surface coatings, and corrosion protection. It can be applied through electroplating, electroless plating, or immersion coating methods to provide functional and decorative finishes on metal parts, automotive components, and consumer goods.
- Ceramics and Glass: Nickel sulfate is used as a colorant and glaze additive in the ceramics and glass industries. It imparts shades of green and gray to ceramic materials and glass products, enhancing their aesthetic appeal and providing decorative effects. Nickel-containing glazes can also improve the durability and chemical resistance of ceramic surfaces.
- Textile Industry: Nickel sulfate is used in the textile industry for dyeing and printing applications. It may be used as a mordant or dye fixative to improve the colorfastness and washfastness of textile dyes on fabrics such as cotton, wool, and silk. Nickel salts can also impart specific colors to textile fibers.
- Chemical Manufacturing: Nickel sulfate serves as a precursor or starting material for the synthesis of various nickel compounds and complex salts. It may be used in the production of nickel catalysts, pigments, chemical intermediates, and specialty chemicals for diverse industrial applications.
- Water Treatment: Nickel sulfate is used in water treatment processes for wastewater remediation and environmental protection. It can be employed in precipitation, coagulation, and flocculation treatments to remove heavy metals, metal ions, and pollutants from industrial effluents and contaminated water sources.
- Research and Development: Nickel sulfate is utilized in research laboratories and academic institutions for experimental studies, chemical synthesis, and material science research. It may be used as a reagent, catalyst, or reference standard in chemical analysis, spectroscopy, and electrochemistry experiments.